An institutional review board (IRB) is a committee or group that evaluates the ethics of a research study or evaluation. IRB approval is usually required for any project involving human participants to ensure the safety and protection of those participants. (IRB approval is particularly important for healthcare studies and studies involving children and other vulnerable populations.) IRBs are often part of universities or research organizations, but they can also be government agencies or private companies.
The purpose of IRB approval “is to make sure that when we do our research and evaluation work, that we adhere to the ethics and principles that uphold people’s rights…but also that there has been an appropriate process thinking through what is the right approach,” says Margie Roper, Khulisa’s Director of Education and Development. “Are our tools fair for everyone to be able to participate in? Have we considered if anyone is being excluded? It’s also making sure that we’ve done no harm to anyone, so that when these results come out, they will be believed.”
The process for obtaining IRB approval can be daunting. But it’s essential, and not just as a box that must be ticked in order to proceed with an evaluation. Navigating IRB approval is an important part of designing and planning a project, and vital for protecting both a study’s participants and its researchers/evaluators.
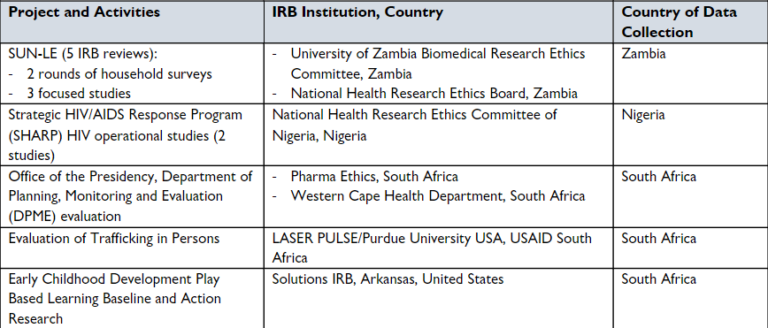
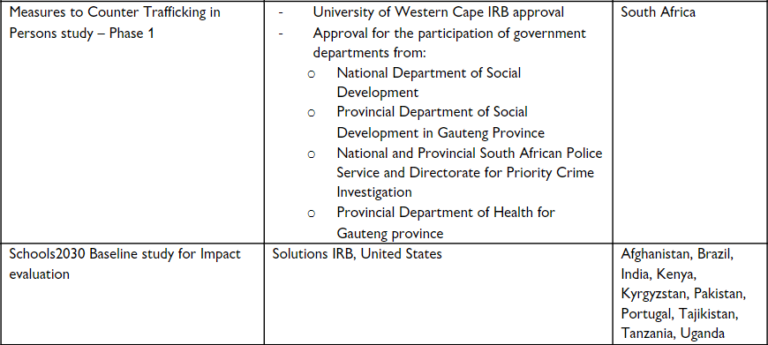
Since 2015, Khulisa has obtained IRB approval for 12 evaluations involving work in 13 different countries, using a variety of IRB institutions. We asked Margie and other Khulisa evaluators who lead these programs to provide tips on how to manage the IRB process, and here’s what they told us:
- Plan ahead.
The IRB process can be long and laborious; it’s essential to plan ahead as far as possible, especially for complex projects. IRB approvals processes differ widely from one institution to the next, but generally take at least three to six months.
It’s important to be clear on why an IRB approval is needed, from whom it is needed , the general timeframe for obtaining approval, and how much it costs. This information has a huge effect on the design and scope of a project. In multi-country or complex evaluations, it’s often necessary to get ethics approvals from multiple boards or organisations , and the approval process could take multiple rounds of questions and responses. Before starting the IRB approval process, think through exactly how the study will be structured and conducted, as knowing this information in advance is essential to the success of the application.
For the Prevalence and Scope of Trafficking in Persons in South Africa study, a large study involving interviews with extremely vulnerable populations, the Khulisa team obtained a preliminary IRB approval during the proposal process. “This meant that as soon as the contract was signed, we could actually start engaging with people and getting data already in the public domain,” Margie says.
Our experience has also shown it’s helpful to speak to or meet with the IRB approval organization before submitting an application, to make sure the requirements are clear and to get as much guidance as possible on how to put the application together. The more preparation you do in advance, the more likely you are to have a smooth approvals process.
- Be aware of costs.
University IRB approvals are more affordable than private IRB institution approvals. (University approval might even be free if the study is taking place within that particular university.) But sometimes using a private institution is necessary. Khulisa’s Schools2030 project, which involves data collection with school children in ten different countries, required a private, international IRB, as well as ethical approvals in each individual country. Each of these approvals had costs associated with them.
- Find the right institution and understand its requirements.
Finding the right IRB in a particular country for the project you’re doing, especially for non-clinical research, may require a bit of research. For projects like Khulisa’s Scaling Up Nutrition Learning and Evaluation (SUN LE) project in Zambia we found the committee we needed by approaching the University of Zambia, which has three different ethics committees covering various research topics.
Once you find the right IRB institution, the next step is to understand that board’s requirements. You may find that an international board or one in a different country requires a very different process to a local IRB you have worked with before. Some private, international IRBs require more information than university IRBs.
Prepare for the possibility that you may need more documentation for the enumerators participating in the research. Depending on where you collect data, you may need translated recruitment materials, consent forms and tools, and documentation of clear processes on how the data privacy will be handled across borders. As mentioned above, it’s best to book a meeting with the IRB team to discuss the application requirements before starting the process.
- Training is important.
Before applying for an IRB approval, make sure all the project’s principal investigators (PIs), country leads, and other relevant project staff receive formal ethics training. Training certification is not only essential for the IRB application process itself, but also to protect both the human subjects in a study and the researchers/staff members doing the work. Working with vulnerable populations like victims of trafficking can be emotionally difficult, even traumatizing, for everyone involved, and researchers need training to cope with those challenges.
Recommended training resources that Khulisa has used include: the CITI program, the Training and Resources in Research Ethics Evaluation (TRREE) and the the Global Health Training Centre. Courses are often (but not always) free.
- Be prepared for changes and problems during the project.
The IRB process doesn’t end once the application is approved. The point of IRB approval is to ensure that systems are in place to deal with changes and problems as they arise over the course of a project; these changes and problems can – and almost always do – happen.
Most evaluations involve a pilot phase, and IRB approval is necessary before conducting that pilot. In many instances the results of the pilot phase necessitate substantial changes to the research tool before the actual fieldwork phase, which in turn necessitates updating the IRB with the revised tools and ensuring these tools remain approved.
When a problem does arise – such as a report of unethical behavior by a data collector, or an injury to a study subject – during the field work phase of a project, it’s important to react quickly both to mitigate the problem and report the problem and its solution to the relevant IRB. It’s best to act immediately to address the problem and then report the issue to the IRB, rather than reporting to the IRB first and then waiting for a response before acting. The protocol for dealing with such problems should already exist in the initial IRB approval, so there is no need to panic.
- Don’t be afraid of the IRB process.
Obtaining IRB approval can be a long, labor-intensive process. But try not to be scared! All the work that you put into an IRB application will pay off in the long run, both for the project at hand and future projects. The IRB process allows evaluators to see their projects through an outside, objective eye, and creates opportunities for constructive feedback.
The process of obtaining IRB approval “has really enhanced all our work,” Margie says. “It’s enhanced the training of our field workers. It’s enhanced our quality assurance surveillance plans. It’s enhanced our informed consent forms, how we engage with clients, our reports…We know that we’ve done the best possible job, and we are confident in what we’re producing.”